
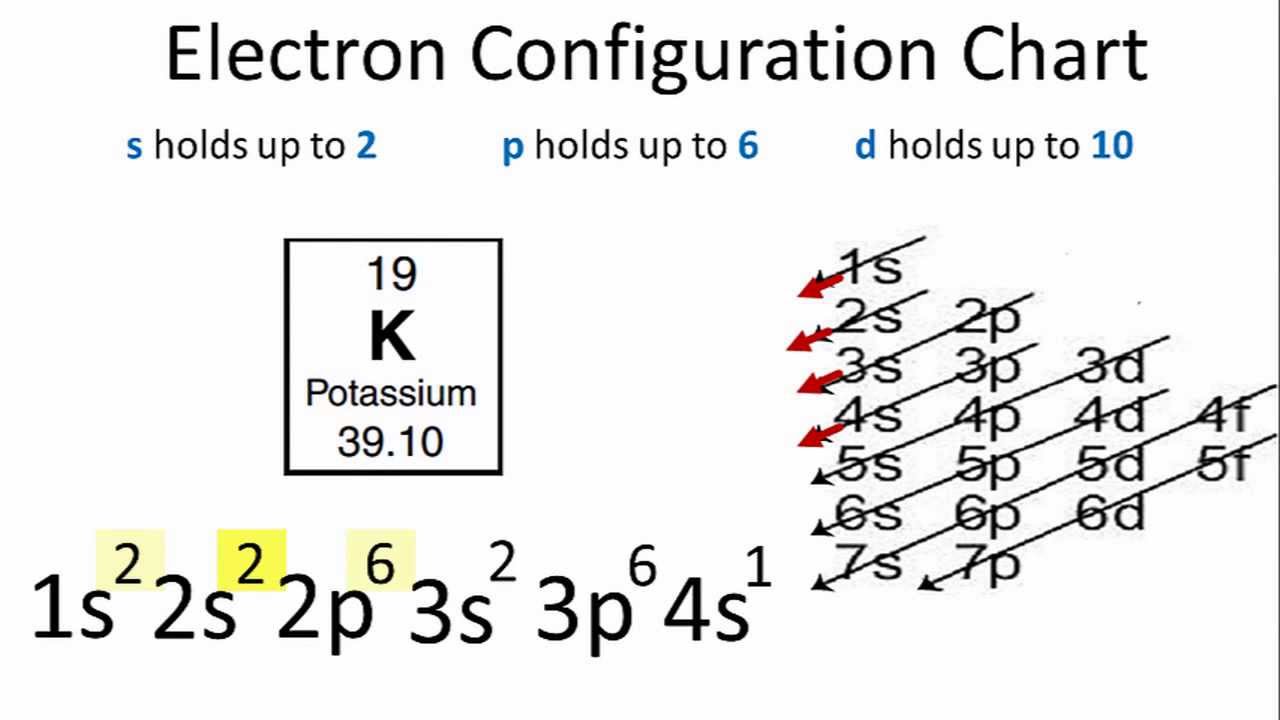
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. Therefore we have 1s 22s 22p 63s 23p 63d 64s 2įor the Fe2+ ion we remove two electrons from 4s2 leaving us with:įor the Fe3+ ion we remove a total of three electrons (two from the 4s2 and one form the 3d6) leaving us with Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written ( here is an explanation why). Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. We find the configuration to be 1s22s22p6 which we know is correct because if you add up the superscripts, you'll get the total number of electrons 10. We continue this path until we reach a total of 10 electrons. We know that the 1s orbital can hold up to a max of 2 electrons. Therefore the Iron electron configuration will be 1s 22s 22p 63s 23p 64s 23d 6. The first way: Neon has a total of 10 electrons.
Ground state electron configuration full#
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. We now shift to the 4s orbital where we place the remaining two electrons. Since the 3s if now full we'll move to the 3p where we'll place the next six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. The p orbital can hold up to six electrons. The traditional argument for why this is so is based on a screening argument that claims. The next six electrons will go in the 2p orbital. The electronic ground state for lithium is 1s22s, and not 1s22p. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. In writing the electron configuration for Iron the first two electrons will go in the 1s orbital.

Video: Fe, Fe 2+, and Fe 3+ Electron Configuration Notation


 0 kommentar(er)
0 kommentar(er)
